
SYMATESE DEVICE provides to professionals in the medical, pharmaceutical and cosmetic surgery sectors with its expertise in the design, development and management of regulatory affairs, industrialisation and marketing of specific administration.

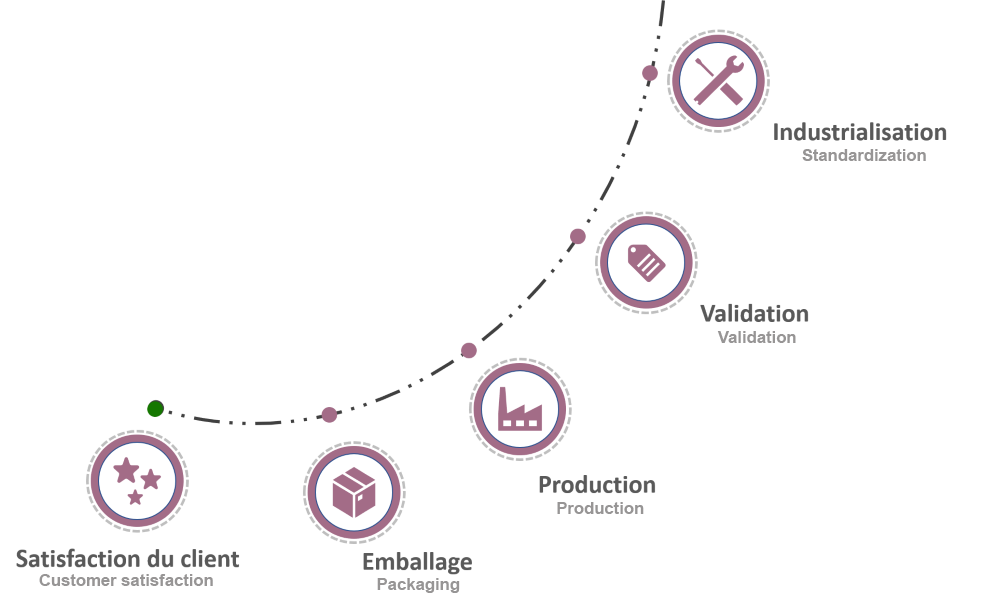
SYMATESE DEVICE is available from the beginning of your creation to support you through the study, design and development phases. The feasibility phase is optimized through the implementation of high-precision functional rapid prototyping.
Throughout this process SYMATESE DEVICE provides the customer with dedicated support in setting up the regulatory file.
An industrialization team takes over for the definition of all the tools and equipment necessary for the realization of the medical device. Rigorous quality monitoring is applied throughout the development process, which ends with a complete validation of equipment and processes according to the three stages of validation: IQ, OQ and QPP. The production and blistering operations carried out 100% in-house represent the last step before marketing to customers.
Copyright © Symatese Device 2023